Special Offer- Buy now and get an
additional 1 hour per month of 1:1 strategy consulting
MDR Navigator
Your Fast Lane to EU Med tech Certification
The world’s first all-in-one platform for fast, efficient, and compliant medical device certification.
MDR Navigator
Your Fast Lane to EU Med tech Certification
The world’s first all-in-one platform for fast, efficient, and compliant medical device certification.
You're Not Alone in Facing MDR Challenges
Regulatory Complexity is Widespread
Complying with MDR regulations is a common struggle across the medtech industry.
Even Experts Face Hurdles
Seasoned professionals encounter significant challenges in staying compliant with ever-evolving MDR requirements.
Universal Impact: From Startups to Giants
Regardless of size, from innovative startups to established corporations, everyone in the medtech field feels the strain of MDR compliance.
Universal Impact: From Startups to Giants
Regardless of size, from innovative startups to established corporations, everyone in the medtech field feels the strain of MDR compliance.
"Mistakes in relation to the MDR can cost companies 100.000€ per month and total delays from several months to multiple years"
Uros Tacar
CEO at TUKO medical devices; Quality Assurance & Regulatory Affairs consultant; Lead auditor
Take the Step Towards Effortless Compliance
Your MDR Certification Journey is just the tip of the Iceberg
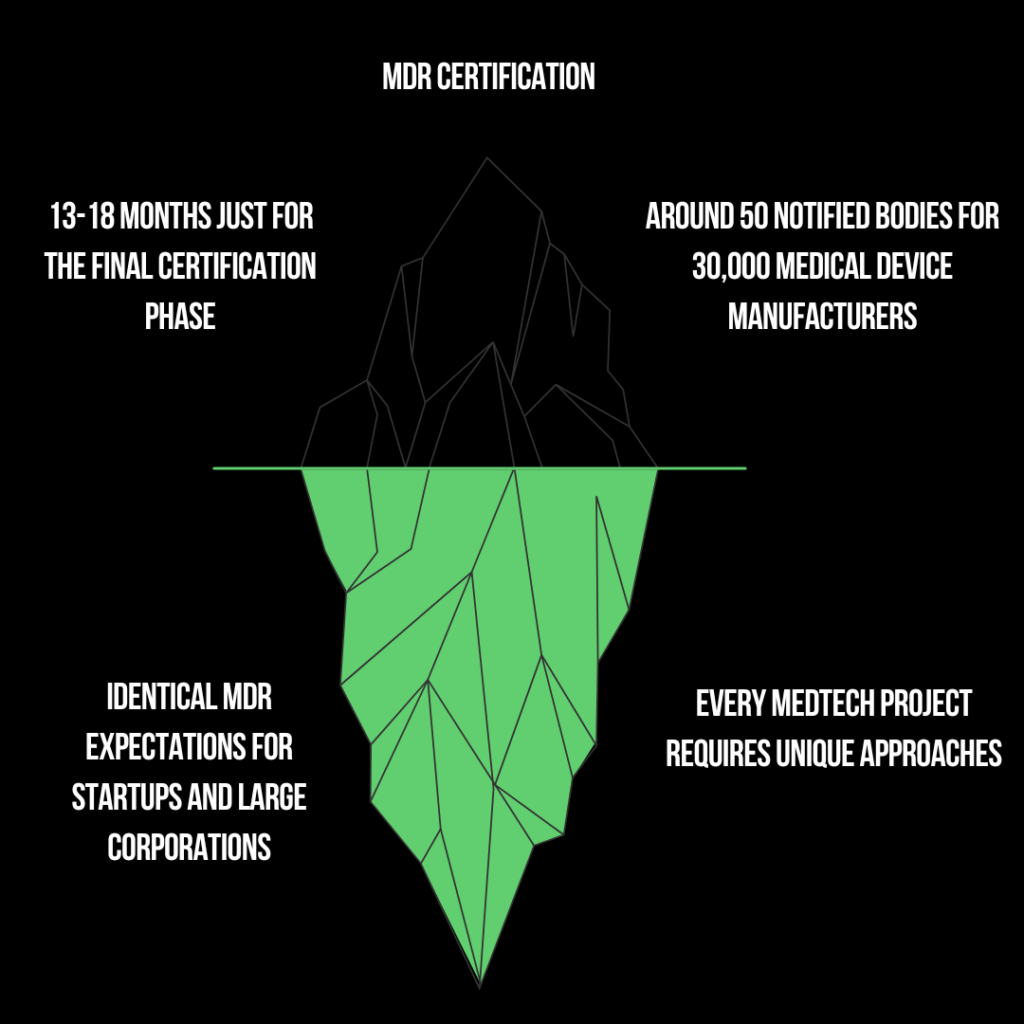
Take Control of Your MDR Compliance Journey
Join the community of MedTech innovators who have accelerated their EU certification.
The Realities of MDR and MedTech Challenges
High Failure Rate:
Startups and projects fail primarily due to regulatory hurdles
Constant Updates:
Frequent updates and additional documents make it a moving target, challenging to keep up with.
Costly Mistakes:
Even minor errors can lead to significant delays and costs, sometimes amounting to hundreds of thousands of euros.
MDR Complexity:
One of the most intricate regulations worldwide, demanding extensive understanding and detailed attention.
Lack of Regulatory Knowledge:
The number one reason for failure is the absence of comprehensive regulatory knowledge.
Underestimating RAQA:
Many companies overlook the importance of integrating RAQA expertise from the beginning, leading to critical gaps.
Take the Step Towards Effortless Compliance
The Realities of MDR and MedTech Challenges
Constant Updates:
Frequent updates and additional documents make it a moving target, challenging to keep up with.
High Failure Rate:
Startups and projects fail primarily due to regulatory hurdles
MDR Complexity:
One of the most intricate regulations worldwide, demanding extensive understanding and detailed attention.
Costly Mistakes:
Even minor errors can lead to significant delays and costs, sometimes amounting to hundreds of thousands of euros.
Lack of Regulatory Knowledge:
The number one reason for failure is the absence of comprehensive regulatory knowledge.
Underestimating RAQA:
Many companies overlook the importance of integrating RAQA (Regulatory Affairs and Quality Assurance) expertise from the beginning, leading to critical gaps.
Take the Step Towards Effortless Compliance
Meet Your Ultimate
MDR Compliance Partner
The world's first all-in-one regulatory Copilot!
A combination of e-learning platform and live support,
to help with the certification of medical devices.
A unique blend of everything a company needs for their MDR journey.
EDUCATION
Experience unparalleled education with our expertly crafted tutorial videos.
Training and learning videos on over 30 different medical device approval topics (new content added continuously)
TOOLS
Unlock a suite of professionally designed templates and efficiency tools.
Over 50 templates that can be freely customized and used for your own project (constant expansion)
SPARRING
Join our weekly live sessions with industry expert Tibor Zechmeister.
Weekly answers to up to 5 relevant questions per unit. In addition, questions can be posted in the forum at any time.
A clear roadmap
From the idea to certification for the entire team.
Ongoing support and guidance
Ensuring that the certification project is on track
The fastest Path
Assurance that the most efficient and fastest path to certification is being used
up to 75% Faster
Certification projects can be accelerated by up to 75%
UP TO 50% Cost reduction
Total project costs can be reduced by up to 50%
Take the Step Towards Effortless Compliance
In which phases can the MDR Navigator be used?
Who is the MDR Navigator suitable for?
Startups and Spinoffs
Start-Ups and Spin-Offs planning their first approval
Research Projects
Research projects that want to develop a medical device from their prototype
Small and medium-sized companies
Small and medium-sized companies that want to train and educate their new regulatory affairs staff
Ready to Simplify Your MDR Journey?
Join the ranks of successful MedTech innovators who trust MDR Navigator for MedTech.
What Our Clients Are Saying




intended Purpose guidance
Describe 95% of relevant topics with the first pass of the intended use template, instead of the usual 20%
Product Classification
Get an initial assessment in 60 minutes instead of 4 weeks with the Medical Device Classification Guide
notified body section
Create a list of potential candidates in less than 2 hours with the Notified Body template instead of searching for 6 months
Explore the Powerful Features of MDR Navigator for MedTech
Step-by-Step Guidance
Our clearly structured platform guides you through each step of the MDR process, ensuring you follow the correct order for optimal results.
Beginner Friendly
Provided information is easy to understand and provides value for both beginners and intermediates, ensuring everyone can navigate MDR successfully.
Interactive Live Sessions
Participate in weekly live sessions where you can ask specific questions and get answers from industry experts in a collaborative group setting.
Externally Validated Resources
A growing collection of tutorial videos and templates, validated by Notified Body Auditors, ensuring top-notch quality and compliance.
Regulatory News and Updates
Stay informed with the latest news and updates about important regulatory changes, so you're always ahead of the curve.
Strictly Filtered Community
Join a dedicated and suitable community of professionals and companies, where only the most committed and relevant members are admitted.
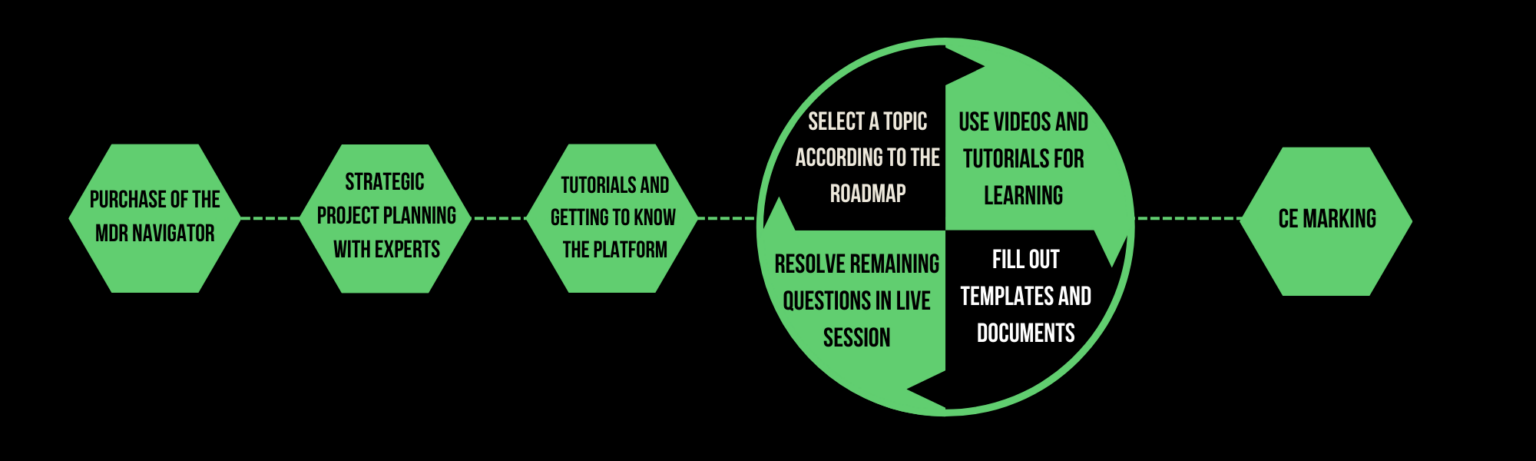
Ready to Revolutionize Your MDR Compliance?
Take the Next Step with the Leading All-in-One Regulatory Platform
Meet Your Regulatory Affairs Expert:
Tibor Zechmeister
Leading Authority in MedTech Compliance and Regulatory Affairs
Extensive Industry Experience
Biomedical engineering background with nearly 15 years of expertise in the MedTech sector.
Successful Entrepreneur:
Founder and builder of 4 innovative companies in the medical device industry.
Accredited Notified Body Audito
Recognized auditor for quality management systems and technical documentation reviews, ensuring compliance excellence.
Published Thought Leader
Author and co-author of scientific literature and expert books; keynote speaker at industry conferences.
Trusted Advisor
Provided regulatory guidance to over 50 startups and large corporations, navigating complex challenges with proven success.
Take the Step Towards Effortless Compliance
Extensive Industry Experience
Biomedical engineering background with nearly 15 years of expertise in the MedTech sector.
Successful Entrepreneur:
Founder and builder of 4 innovative companies in the medical device industry.
Accredited Notified Body Audito
Recognized auditor for quality management systems and technical documentation reviews, ensuring compliance excellence.
Trusted Advisor
Provided regulatory guidance to over 50 startups and large corporations, navigating complex challenges with proven success.
Published Thought Leader
Author and co-author of scientific literature and expert books; keynote speaker at industry conferences.
Take the Step Towards Effortless Compliance
ON Point EDUCATION
POWERFUL Templates
Live Sparring



FREQUENTLY ASKED QUESTIONS
MDR Navigator for MedTech is an all-in-one regulatory platform designed to help medical device manufacturers navigate the complex European Medical Device Regulation (MDR) efficiently and compliantly.
Our platform provides externally validated resources, including tutorial videos and templates, step-by-step guidance, and regular updates on regulatory changes to ensure you meet all MDR requirements.
MDR Navigator for MedTech is designed for startups, small businesses, and large corporations in the medical device industry seeking efficient and compliant MDR certification.
Unlike traditional consulting or workshops, our platform offers 24/7 availability, a comprehensive step-by-step guide, ongoing expert support, and a strictly filtered community of dedicated professionals.
Getting started is easy! Simply book a free consultation through our website to discuss your needs and see how our platform can support your regulatory journey.
You will have access to weekly live sessions with regulatory experts, a rich library of validated resources, and continuous updates on regulatory changes to ensure you stay compliant.
Yes, our platform is designed to be user-friendly and provides value to both beginners and intermediates, making the complex MDR process easier to understand and navigate.
We prioritize the security of your data with robust measures to protect your sensitive information throughout the compliance process.
The cost of MDR Navigator for MedTech is much lower than traditional 1:1 consulting, providing exceptional value for comprehensive regulatory support.
Our resources and templates are regularly updated to reflect the latest regulatory changes and best practices, ensuring you always have the most current information.
You can book a free consultation to learn more about how our platform can benefit your regulatory process and see if it’s the right fit for your needs.
Our platform is specifically designed for the medical device industry, catering to manufacturers seeking to comply with European MDR regulations.
The Navigator was created in a way that traditional 1:1 consulting is not necessary anymore. Our comprehensive platform provides all the support and resources you need to achieve compliance efficiently.
We are convinced that the quality and value of our platform will impress you. However, if you are not satisfied, we offer a full refund within the first month.
After the consultation, we will evaluate whether the project can benefit from the MDR Navigator and whether the team is a good fit for the community. In the event of a positive assessment, an individual offer for the collaboration will be drawn up.
Use can be started within 2 weeks of a positive evaluation. In the preparatory weeks, there is a personal strategy meeting for the roadmap and an introduction to the tool.
Yes, after the free consultation, a section of the MDR Navigator can be tested free of charge before a decision is made.
